By Abdelhanine Ayada, Omar Bessebouab, Sofiane Aissanoua, Monika Stefaniuk-Szmukierc, Katarzyna Piórkowskac, Adriana D. Musiałc, Boguslawa Długoszd, Agnieszka Kozłowskac, Katarzyna Ropka-Molikc
Genetic disorders are recognised as hereditary diseases with the most significant economic impact on horse breeding, causing important foal losses, costs of treatments of horses, and maintenance of the mare during the pregnancy.
Selle Francais horses are recognized in many countries and are showing great results in equestrian sports around the world (dressage, showjumping, and eventing). This study aimed to detect the presence of three mutant alleles associated with inherited diseases including Fragile Foal Syndrome (FFS), Cerebellar Abiotrophy (CA), Polysaccharide Storage Myopathy (PSSM1) and variant impacting gait type in DMRT3. This trait is important for breeding decision in Selle Francais horses and sheds new light on genetic potential and risks on this breed. The genotyping was performed on 91 Selle Francais horses using PCR-RFLP (for POLD1; GYS1 and DMRT3 genes) and PCR-ACRS (TOE1 gene) methods. The presented report indicated the presence of mutant allele A casual for PSSM1 and allele T associated with FFS syndrome occurrence, in 4% and 6% of analysed horses, respectively. Regarding CA, the present survey did not register any cases of this genetic disorder in Selle Francais horses. Our results show also that about 1% of all the Sell Francais horses studied carry the A allele of DMRT3 gene. The present findings have provided data for these fulness of monitoring genetic diseases and gait type in the investigated breed to avoid losses of offspring.
1. Introduction
The Selle Francais is an example of a Warmblood horse, in which origin backs to local noble mares from Normandy crossed with Thoroughbred (TB) or Norfolk stallions [1]. In the 19th century in France, the presence of three main types of half-blood horses was recorded: Anglo-Norman (bred around Caen), the demi-sang du Centre (bred around Cluny), and the Vendéen (bred around La Roche-sur-Yon) [2].
To further excel in equestrian sport, such as show jumping and dressage in 1958, several local French horses were merged under the one studbook for Cheval de Selle Francais (SF). The quality of the horses was confirmed by further use of SF in improvement in other breeds like the Holsteiner (via Cor de la Bryère), the Zangersheide (stallion Almé), or the Oldenburg (stallion Furioso).
Furthermore, the SF breed gained higher number of active Warmblood stallions used in different studbooks [3], which was related to modern breeding techniques allowing for intensive international exchange of breeding stock and finally the progress in the further specialisation of these breeds [4]. It was reported that the distribution of the number of offspring, male or female, per stallion is unbalanced in some breeds, particularly for the Selle Français.
The average number of useful male offspring per stallion with at least one useful male offspring ranged from 2.1 in Arab to 3.9 in Selle Fracais [5]. Some horse origins are actually defined by the fact that they are constituted by first generation crossbred individuals, such as Half Bred Arab origin. It also has to be underlined that a limited part of horses raised in France do not belong to any specific breed. Another study concluded that the Thoroughbred constitutes by far the main origin for the Selle Français and Anglo-Arab breeds (46.7 and 54.4% of founder origins, respectively) [6].
Almost 30 years of intensive research on the genetic background of disorders and important traits created powerful selection tools. Genetic disorders are recognised as hereditary diseases with the most significant economic impact on horse breeding, causing important foal losses, costs treatments of horses, and maintenance of the mare during the pregnancy. Inheritance may be recessive and a carrier of the disease-causing allele shows no clinical signs (as Fragile Foal Syndrome - FFS and Cerebellar Abiotrophy - CA disorders).
In turn, some of the disorders, as Polysaccharide Storage Myopathy 1 (PSSM1), present the dominant model of inheritance and only one copy of the mutant allele is enough for disease symptoms to appear. Genetic disorder of the recessive model of inheritance allows to spread the unfarmable alleles relatively quickly in the population, due to the lack of symptoms in carries horses, and the increase the possibility to the birth of affected foals. While CA appears to be almost entirely restricted to the Arabian breed, Arabian horses have contributed to the genetic make-up of many other breeds and it is therefore possible that the CA mutation could be present in other breeds including Selle Francais [7].
It is essential to continually evaluate breeding stock by genetic testing to avoid and prevent the birth affected foals effectively. Such information is interesting and informative for equine practitioners to reduce the risk of homozygous individuals and remove carriers from the breeding population. Thus, the study aimed to detect the presence of some mutant alleles associated with inherited diseases such as FFS, CA, and PSSM1 that can be important for breeding decision in Selle Francais horses and sheds new light on genetic potential and risks on this breed. According to the previous literature data [8], FFS was already tested in the SF breed, however, due to the seriousness of this disease, a larger group of horses additionally from different geographical regions was tested in the present report. The Warmblood sport horse should stand out with excellent gait quality and are bred worldwide with the aim to compete in the Olympic sports as such jumping, dressage, and eventing. Many authors reported that the success of the sport horse industry is based essentially on implementing successful breeding programs and the genetic improvement of the sport horse, including the effect feeding or management, and the effect of genetic gain are cumulative and permanent.
The Selle Français breed is the breed of riding horse and widespread around the world, and is bred primarily for show jumping, but also for dressage and cross-country. Therefore, we believe that this aspect is important to remember and to make the link between equestrian sports performance and genetic diseases that transmit any allele likely to cause significant dysfunctions in sport horses. The premature stop codon within DMRT3 mutation is a candidate gene affecting locomotion pattern and is influential in harness racing [9]. However, in breeds used in sport competition such as jumping, or dressage, the altered gait is recognised as the fault. Thus, the study aimed to detect the presence of the mutant allele in DMRT3 to determine if this allele was present and if so if it could impact breeding decision in Selle Francais.
2. Materials and methods
2.1. Sampling: Selle Francais horses’ population from Algeria, Poland, and France was chosen randomly (n=91) for each of the three genetic disorders and genetic gait type determination. Thirty hairs approximately 7cms long were collected out at the root of the neck or tail skin. The obtained material was preserved in an individually labelled paper envelope stored at room temperature until further analysis. All animals involved in this study were checked clinically by a veterinarian as healthy. Without suspicion of disease, and presented no signs of neurologic disease and incorrectness of gait.
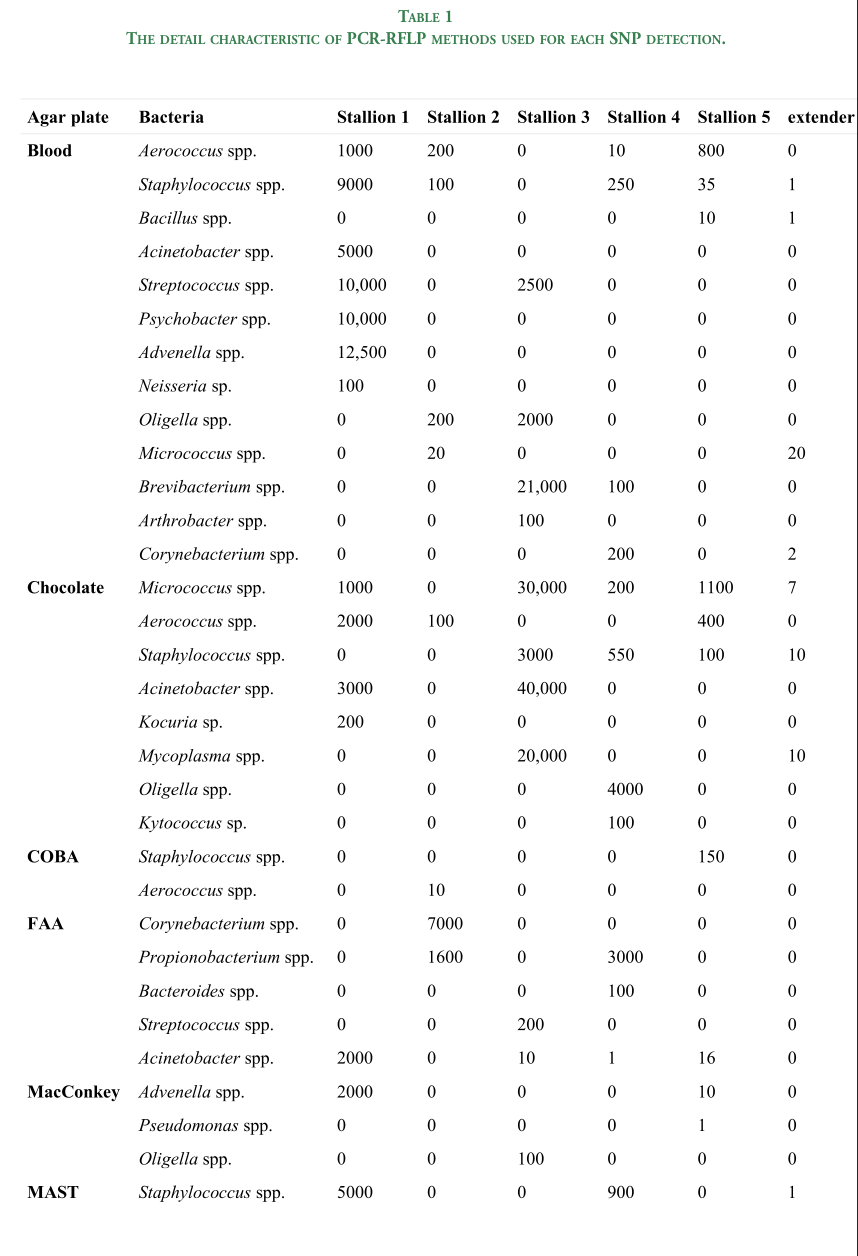
Pictures of agarose gel electrophoresis of three polymorphisms detected in Selle Francais horses (M - DNA Marker 2; A&A Biotechnology). The exact length of presented alleles is presented at Table 1
The neurologic examination evaluates: (1) the cranial nerves, (2) the gait, or walk, (3) the neck and front legs, and (4) the torso, hind legs, anus, and tail. None of the horses showed signs of lowering reflexes of the head deterioration, constant pacing, seizures, a head turn or circling in one direction or other unusual head movements. Also, horses showed no signs of dysfunction within gait evaluation such as circling, weakness or complete paralysis of any limbs, falling, stumbling, rolling, or loss of coordination.
For evaluation of neck and front legs there were no evidence of pain, loss of muscle tone or cramp in the neck. Likewise, none signs of loss of feeling or hypersensitivity to light touch or pinpricking, and loss of muscle mass have been shown in horses.
2.2. DNA isolation and genotyping: Genomic DNA was extracted from all samples using the Sherlock AX kit (A&A Biotechnology, Poland) according to protocol. The DNA quality and concentration were measured with NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The DNA samples with concentraiton 100ng/ul were used to furhter analyses.
2.3. Polysaccharide storage myopathy (PSSM1): A polymorphism in the GYS1 gene related to Polysaccharide storage myopathy (PSSM1) was identified using the PCR-RFLP method. The primers were designed based on ENSECAG00000021428 reference and amplified the 282 bp of GYS1 gene (Table 1; Fig. 1). The PCR reaction was performed using AmpliTaq Gold™ 360 Master Mix (Applied Biosystems, Thermo Fisher Scientific,Waltham, MA, USA) according to protocol and at 57°C of annealing temperature. After endonuclease digestion, two alleles were obtained: G - 202 and 80bp and A - 148, 80, 54, and 19bp.
2.4. Fragile Foal Syndrome Type 1 (FFS): To identify polymorphism related to Fragile Foal Syndrome Type 1 (FFS), the PCR-RFLP method was used. The missense variant within PLOD1 locus was detected (rs1136065234; NC_009145.3:g.39927817C>T;ENSECAP00000020673. 1:p.Gly678Arg) FauI endonuclease (BioLabs, New England, Poland) (Table 1; Fig. 1) [8]. The obtained products for alleles were as follows: C – 102 and 64 bp; T – 166bp.
2.5. Cerebellar abiotrophy (CA): The missense polymorphism (rs397160943,NC_009145.3:g.13122415C>T; ENSECAP00000020698.1:p.Arg95His) associated with CA occurrence was identified using the PCR-ACRS method. The amplicon spans the fragment of TOE1 gene (ENSECAG00000023204) was amplified using 2 × Phanta Max Master Mix (Vazyme, Polgen, Poland) according to protocol. After endonuclease digestion with HpyCH4III (BioLabs, NewEngland, Poland) the products were obtained as follows: C allele - 111, 22 bp, and T allele -133bp (Table 1). 2.6. Gait type ability (DMRT3 gene) The stop gain mutation of the DMRT3 (rs1150690013;NC_009166.3:g. 22391254C>A; ENSECAP00000020624.3:p. Ser464Ter) gene was identified and genotyped using the PCR-RFLP method.
The primers were designed using Primer3 Input (version 0.4.0) and ENSECAT00000024805 reference (Table 1; Fig. 1). After endonuclease digestion, two alleles were obtained as follow: C – 242bp; A - 166 and 76bp. For each of analyzed genes, the negative and positive samples (heterozygote horses for each disorder) were included in every analysis. The genotypes were previously confirmed using Sanger sequencing an based on reference sequences. For each set of PCR the no-template control (NTC) reactions were performed.
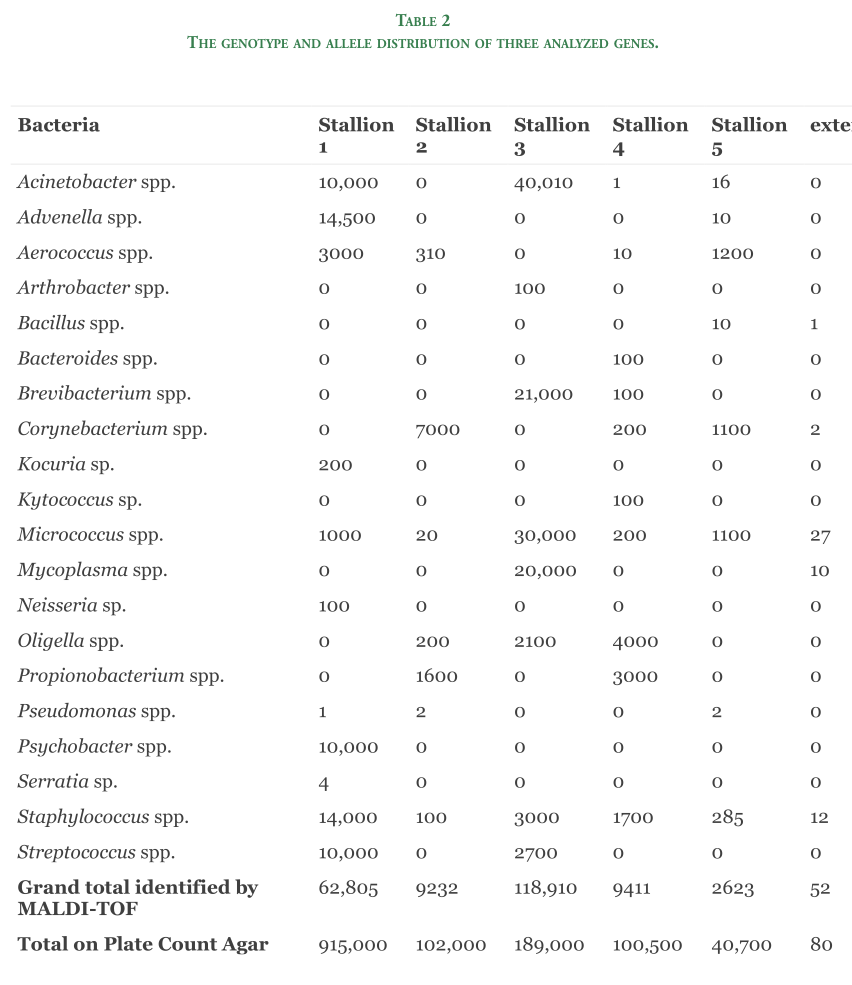
The genotype and allele distribution of three analyzed genes.
3. Results and discussion
The SF horses are a model example for over 50 years, mainly focused on showjumping. To achieve an excellent sport horses French breeding scheme, we should establish selection procedures with particular traits [4]. Besides the main goal, most breeders use information about gaits, conformation, behaviour, fertility, and health [10]. The genetic selection has impacted the economy of horse breeding and breeders searching for tools with great potential. Genetic tests allow monitoring the genetic disorders variants, variations within coat colour and alleles affecting important traits such as gait type, exterior, and behavior.
Within the presented study, 91 Selle Francais horses were genotyped in terms of the presence of mutations associated with the three hereditary diseases and alleles involved in the occurrence of amble gait. The presented report indicated the presence of mutant allele A related to Polysaccharide Storage Myopathy in 2%, which was represented by heterozygote horses in the amount of 4% (Table 2). PSSM1 is an autosomal dominant glycogen storage disorder that occurs when mutation G>A in the glycogen synthase 1 gene (GYS1), turns arginine amino acid (CGT) in the 309th position in the GYS1 gene into histidine (CAT).
As the glycogen synthase catalyses the polymerisation of glucose from a UDP-glucose (UDPG) substrate via α1-4 glycosidic bonds [11] the mutated allele results in increased glycogen synthase activity in muscles. That results in excessive amounts of muscle glycogen and amylopectin accumulation via constitutive enzyme activation [12].
The clinical symptoms of PSSM1 appear in forms of exertional rhabdomyolysis, muscle atrophyand soreness, gait abnormalities with severe muscle pain and myoglobinuria are seen in warmblooded horses, draft horses, Quarter horses and other breeds [12,13,14,15,16].
The presented data show the appearance of A allele also in Selle Francais breed, which strongly indicates the need of genetic testing and monitoring of parental populations. According to American Association of Equine Practitioners, to date only the Type 2 PSSM (PSSM2) occurrence has been reported in Selle Francais horses.
The horses affected with the symptoms of PSSM2 showed similar symptoms thoses PSSM1, the affected horses do not present the GYS1 mutation (https://aaep.org/). The results obtained indicated on the possibility of both Polysaccharide Storage Myopathy 1 and 2 occurrence in Selle Francais horses, which additionally strengthens the need for genetic testing to diagnose which type of disease has been identified.
One of the most recent established genetic disorders within the Warmbloods is FFS, disease involving a defect of connective tissue and foals born with hyper-extendible, abnormally thin, fragile skin that rips easily and similar to Ehlers-Danloess Syndrome (EDS) in humans [17] and other mammal species [18,19]. It is proposed to be an autosomal recessive disorder caused by a single nucleotide variant in the procollagen-lysine-2-oxoglutarate-5-dioxygenase 1 – PLOD1 gene [20].
The Warmblood Fragile Foal Syndrome (WFFS) has been first studied in Warmblood horses, but according to recent findings, PLOD1 allele occurred not only in Warmblood breeds and thus, it has been postulated to rename FFS [21]. In the present study, tested Selle Francais were heterozygotes for the PLOD1 allele in a percentage of 6% - CT and the mutant allele sowed the frequency 3% (Table 2).
According to SF stud book, about 7,000 foals are born each year, thus, combined with presented allele frequency, around 420 foals might carry out the mutated alleles annually. We did not detect any affected homozygotes, which can result from the low frequency of mutant allele or, as showed in previous reposts, homozygous form of PLOD1 g.39927817C>T can be lethal [22,23].
On the first hand, in the Thoroughbred population, authors showed that despite the occurrence of T allele in investigated breed, the affected foals weren’t observed [23]. It was hypothesised that presence of both TT alleles can result in early embryonic loss [23].
On the other hand, the most recent study allows to show the first fragile foal syndrome in the Thoroughbred foal caused by PLOD1 c.2032G>A [24]. The authors eliminated any other genetic basis of collagen dysfunction besides the PLOD1 mutation [24].
The FFS heterozygous Warmblood sport horses have been reported in many countries specialising in Warmblood horses, e.g. in Germany [25], Brazil [22] and Sweden [26]. The recent worldwide study showed 5.77% share of a deleterious variant in SF breed [8], which is similar to results obtained in present report and confirmed the need of genetic testing implementation in breeding decision.
Historical sources indicate the addition of Anglo-Arabian horses in shaping the SF breed; thus we perform screening for alleles involved in Cerebellar abiotrophy (CA), which is a progressive neurological disease characterised by the degeneration of cerebellar Purkinje cells, which affects several animal species [27,28].
It is demonstrated that CA is found almost exclusively in the Arabian breed [29]. However, the probability that mutation CA is present in other breeds, because the Arabian horses have contributed to the genetic composition of many other breeds [30].
Regarding Cerebellar abiotrophy (CA), the present survey did not register any cases of this genetic disorder in Selle Francais horses. Additionally, a genetic marker related to gait type was also evaluated. The DMRT3 (doublesex and mab-3 related transcription factor 3) was established to affect locomotion in horses [9]. The role of this gene on a specific subset of spinal cord neurons was demonstrated in mice.
In horses, the mutation (cytosine C to adenine A), initially discovered in Icelandic horses, causes a premature stop codon and thus a truncation of the DMRT3 protein. Our results show that one horse (about 1%) of all the Selle Francais horses studied carry the A allele of DMRT3 gene.
Numerous studies reported that the mutant allele A of g.22999655C>A DMRT3 SNP is the most frequent in gaited horses [9,31,32]. Moreover, allele A has been associated with good quality of pace and gait when compared to animals with genotype CC [9,33]. Therefore, it seems important to evaluate the DMRT3 genotypes in Selle Francais horses since gait is one of the criteria determining the stud-books registration, and this breed can achieve success in dressage and eventing.
4. Conclusion
To our knowledge, this is the first report focused exclusively on genetic screening in Selle Francais horses which is recognized in many countries and is showing great results in equestrian sports around the world (dressage, showjumping and eventing). The present findings have provided data for the usefulness of genetic diseases monitoring in the investigated breed to avoid losses of offspring.
Furthermore, our findings indicate the occurrence of A allele increasing the predisposition to natural ability for pace, excellent leg coordination and trotting technique at high speed. In future, the genetic monitoring of alleles related to genetic disorder occurrence should be investigated on large number of horses and different population which can differ in the terms of unfavourable allele frequency.
First published in the Journal of Equine Veterinary Science, Volume 116, September 2022 This research was approved by the Scientific Council of the Faculty of Nature and Life Sciences (Report of Faculty Scientific Council #05 dated November 11, 2020), University of Bejaia, Algeria). Concerning the ethical aspects, the experimental procedure was performed according to good veterinary practice under farm conditions. Study supported by the National Research Institute of Animal Production, Krakowska 1, 32-083 Balice, Poland
This research was approved by the Scientific Council of the Faculty of Nature and Life Sciences (Report of Faculty Scientific Council #05 dated November 11, 2020), University of Bejaia, Algeria). Concerning the ethical aspects, the experimental procedure was performed according to good veterinary practice under farm conditions.
References
[1] EH. Edwards. Les chevaux (in French) Éditions de Borée (2006), pp. 72-73
[2] https://equipedia.ifce.fr/elevage-et-entretien/race-et-robe/races-dequides-et-stud-book/selle-francais
[3] EPC Koenen, LI Aldridge, J. Philipsson. An overview of breeding objectives for Warmblood sport horses; Livest Prod Sci, 88 (1-2) (2004), pp. 77-84
[4] C Dubois, A Ricard. Efficiency of past selection of the French Sport Horse: Selle Français breed and suggestions for the future; Livest Sci, 112 (1-2) (2007), pp. 161-171
[5] S Moureaux, E Verrier, A Ricard, JC Meriaux. Genetic variability within French race and riding horse breeds from genealogical data and blood marker polymorphisms; Genet Select Evol, 28 (1) (1996), pp. 83-102
[6] P Pirault, S Danvy, E Verrier, G. Leroy. Genetic structure and gene flows within horses: a genealogical study at the French population scale; Plos one, 8 (4) (2013), p. e61544
[7] LS Brault, MCT. Penedo. The frequency of the equine Cerebellar abiotrophy mutation in non-Arabian horse breeds; Equine Vet J, 43 (6) (2011), pp. 727-731
[8] S Reiter, B Wallner, G Brem, et al. Distribution of the Warmblood Fragile Foal Syndrome Type 1 Mutation (PLOD1 c.2032G>A) in different horse breeds from Europe and the United States; Genes, 11 (2020), p. 1518
[9] LS Andersson, M Larhammar, F Memic, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice; Nature, 488 (2012), pp. 642-646
[10] M Kulisa, B Długosz, M Pieszka, J Łuszczyński, J. Kochan. Evaluation trial of Trotters breeding in Poland; Biotechnol Anim Husb, 22 (1-2) (2006), pp. 127-131
[11] A Buschiazzo, JE Ugalde, ME Guerin, W Shepard, RA Ugalde, PM. Alzari. Crystal structure of glycogen synthase: homologous enzymes catalyze glycogen synthesis and degradation; EMBO J, 23 (16) (2004), pp. 3196-3205
[12] B Herszberg, ME McCue, T Larcher, et al. A GYS1 gene mutation is highly associated with polysaccharide storage myopathy in Cob Normand draught horses; Anim Genet, 40 (2008), pp. 94-96
[13] ME McCue, WP Ribeiro, SJ. Valberg. Prevalence of polysaccharid storage myopathy in horses with neuromuscular disorders; Equine Vet J, 36 (2006), pp. 340-344
[14] ME McCue, SJ Valberg, MB Miller, et al. Glycogen synthase (GYS1) mutation causes a novel skeletal muscle glycogenosis; Genomics, 91 (5) (2008), pp. 458-466
[15] AM Firshman, SJ Valberg, JB Bender, CJ. Finno. Epidemiologic characteristics and management of polysaccharide storage myopathy in Quarter Horses; Am J Vet Res, 64 (10) (2003), pp. 1319-1327
[16] BA Valentine, SP McDonough, YF Chang, AJ. Vonderchek. Polysaccharide storage myopathy in Morgan, Arabian, and Standardbred related horses and Welsh-cross ponies; Vet Pathol, 37 (2) (2000), pp. 193-196
[17] H Yeowell, S. Pinnell. The Ehlers-Danlos syndromes; Semin Dermatol, 12 (1993), pp. 229-240
[18] KA Holbrook, PH Byers, DF Counts, GA. Hegreberg. Dermatosparaxis in a Himalayan cat: II. Ultrastructural studies of dermal collagen; J Invest Dermatol, 74 (1980), pp. 100-104
[19] K Barnett, BD. Cottrell. Ehlers-Danlos syndrome in a dog: ocular, cutaneous and articular abnormalities; J Small Anim Pract, 28 (1987), pp. 941-946
[20] NJ. Winand. Identification of the causative mutation for inherited connective tissue disorders in equines and methods for testing for same; US Patent Appl (No 14/117) (2014), p. 969
[21] K Martin, S Brooks, M Vierra, WT Lafayette, S McClure, M Carpenter, et al. Fragile Foal Syndrome (PLOD1 c.2032G>A) occurs across diverse horse populations; Anim Genet, 52 (2021), pp. 137-138
[22] M.N Dias, Abranches de Andrade DG, AR Teixeira-Neto, et al. Warmblood Fragile Foal Syndrome causative single nucleotide polymorphism frequency in Warmblood horses in Brazil; Vet J, 248 (2019), pp. 101-102
[23] RR Bellone, NR Ocampo, SS Hughes, V Le, R Arthur, CJ Finno, MCT. Penedo. Warmblood fragile foal syndrome type 1 mutation (PLOD1 c.2032G>A) is not associated with catastrophic breakdown and has a low allele frequency in the Thoroughbred breed; Equine Vet J, 52 (3) (2020), pp. 411-414
[24] AS Grillos, JM Roach, AM de Mestre, AK Foote, NB Kinglsey, MJ Mienaltowski, RR. Bellone. First reported case of fragile foal syndrome type 1 in the Thoroughbred caused by PLOD1 c.2032G>A; Equine Vet J, 00 (2022), pp. 1-8
[25] C Aurich, S Müller-Herbst, W Reineking, et al. Characterization of abortion, stillbirth and non-viable foals homozygous for the Warmblood Fragile Foal Syndrome; Anim Reprod Sci, 211 (2019), Article 106202
[26] M Ablondi, S Eriksson, S Tetu, A Sabbioni, Å Viklund, S. Mikko. Genomic divergence in Swedish Warmblood horses selected for equestrian disciplines; Genes, 10 (12) (2019), p. 976
[27] A. de Lahunta. Abiotrophy in domestic animals: a review; Can J Vet Res, 54 (1) (1990), pp. 65-76
[28] EY Scott, KD Woolard, CJ Finno, JD. Murray. Cerebellar abiotrophy across domestic species; Cerebellum, 17 (3) (2018), pp. 372-379
[29] A Blanco, R Moyano, J Vivo, et al. Purkinje cell apoptosis in Arabian horses with Cerebellar abiotrophy; J Vet Med A, 53 (2006), pp. 286-287
[30] LS Brault, MCT. Penedo. The frequency of the equine Cerebellar abiotrophy mutation in non-Arabian horse breeds; Equine Vet J, 43 (6) (2011), pp. 727-731
[31] M Promerová, LS Andersson, R Juras, MCT Penedo, et al. Worldwide frequency distribution of the “Gait keeper” mutation in the DMRT3 gene; Anim Genet, 45 (2014), pp. 274-282
[32] L Patterson, EA Staiger, SA. Brooks. DMRT3 is associated with gait type in Mangalarga Marchador horses, but does not control gait ability; Anim Genet, 46 (2015), pp. 213-215
[33] K Jäderkvist, L Johansson, A Mykkänen, M Mäenpää, et al. The DMRT3 “Gait keeper” mutation affects harness racing performance and riding traits in Finnhorses; J Equine Vet Sci, 5 (35) (2015), p. 399
[/s2If]





