By Authors listed at the end of this article Photography/graphics:
The cloning of horses is a commercial reality, yet the availability of oocytes for cloned embryo production remains a major limitation. Immature occytes collected from abattoir-sourced ovaries or from live mares by ovum pick-up (OPU) have both been used to generate cloned foals.
However, the reported cloning efficiencies are difficult to compare due to the different somatic cell nuclear transfer (SCNT) techniques and conditions used. The objective of this retrospective study was to compare the in vitro and in vivo development of equine SCNT embryos produced using oocytes recovered from abattoir-sourced ovaries and from live mares by OPU. A total of 1,128 oocytes were obtained, of which 668 were abattoir-derived and 460 were OPU-derived.
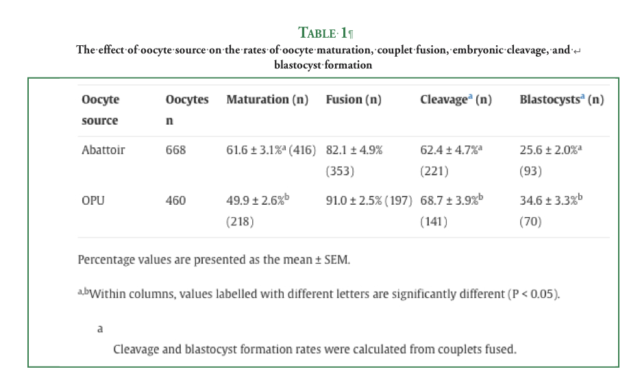
The methods used for in vitro maturation and SCNT were identical for both oocyte groups, and the embryos were cultured in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham medium supplemented with 10% fetal calf serum. Embryo development in vitro was assessed, and Day 7 blastocysts were transferred to recipient mares. The embryos were transferred fresh when possible, and a cohort of vitrified-thawed OPU-derived blastocysts was also transferred. Pregnancy outcomes were recorded at Days 14, 42, and 90 of gestation and at foaling. The rates of cleavage (68.7 ± 3.9% vs 62.4 ± 4.7%) and development to the blastocyst stage (34.6 ± 3.3% vs 25.6 ± 2.0%) were superior for OPU-derived embryos compared with abattoir-derived embryos (P < 0.05). Following transfer of Day 7 blastocysts to a total of 77 recipient mares, the pregnancy rates at Days 14 and 42 of gestation were 37.7% and 27.3%, respectively. Beyond Day 42, the percentages of recipient mares that still had a viable conceptus at Day 90 (84.6% vs 37.5%) and gave birth to a healthy foal (61.5% vs 12.5%) were greater for the OPU group compared with the abattoir group (P < 0.05).
Surprisingly, more favourable pregnancy outcomes were achieved when blastocysts were vitrified for later transfer, probably because the uterine receptivity of the recipient mares was more ideal. A total of 12 cloned foals were born, 9 of which were viable. Given the differences observed between the two oocyte groups, the use of OPU-harvested oocytes for generating cloned foals is clearly advantageous. Continued research is essential to better understand the oocyte deficiencies and increase the efficiency of equine cloning.
1. Introduction
Twenty years ago, the first horse foal cloned from somatic cells was born[1]. The possibilities that somatic cell nuclear transfer (SCNT) presented for disseminating, perpetuating, and salvaging the genetics of rare and valuable horses were immediately obvious. The cloning of exceptional geldings would facilitate the use of the resulting male foals as breeders once sexually mature. Likewise, SCNT would allow the replication of champion mares that have no breeding opportunities during their most fertile years due to demanding competition schedules.
Further, the genetic reconstitution of unbred individuals from biopsied tissue would now be possible following unexpected or accidental death. Despite the SCNT inefficiencies and associated challenges particular to equids, early studies showed that most cloned foals developed normally, and horse clone production was soon commercialised[2]. Many hundreds of horse clones have now been produced around the world, and the SCNT procedures have been refined to the stage where they can be readily applied in equine clinical practice[3,4].
While the equine cloning achievements to date are remarkable, the efficiency of SCNT remains low, with losses at each step of cloned embryo production and throughout gestation following embryo transfer. Hence, as large numbers of oocytes are needed to produce a cloned foal, a major constraint of equine SCNT is the supply of oocytes[5]. One source of immature oocytes that has been used widely by researchers to produce foals by SCNT and intracytoplasmic sperm injection (ICSI) involves the post-mortem recovery of ovaries from slaughtered mares[1,6,7,8, 9,10,11]. Extensively flushing and scraping the antral follicles of ovaries collected post-mortem has been reported to yield a mean of 14 oocytes per mare[12], though typically about 4 cumulus-oocyte complexes (COCs) are recovered per abattoir-sourced ovary[8]. A regular and reliable supply of post-mortem ovaries is often difficult to gain access to, and horse abattoirs are scarce in most countries. In Australia, small numbers of mares are euthanized sporadically, and the importation of abattoir-derived oocytes is not a viable option due to biosecurity issues[13]. Furthermore, in some countries, like the United States, the slaughter of horses is banned[14].
Immature oocytes have also been collected from live mares by transvaginal ultrasound-guided follicle aspiration, or ovum pick-up (OPU) to produce cloned foals[15],[16],[17]. The use of oocytes from the same maternal line as the nucleus donor animal avoids the presence of heterogenous mitochondrial DNA in the foal[15]. Although OPU is costly and technically challenging in mares, and there is some risk associated with the procedure, this oocyte source is increasingly being used for the production of ICSI embryos [18]. With the various refinements made to the equine OPU technique[19], experienced practitioners can now recover a mean of 9-14 oocytes per mare following aspiration of antral follicles 6-30mm in diameter[20],[21],[22]. Mare age, breed and season have been found to influence the recovery of oocytes by OPU[20,23].
Following the collection and transportation of immature oocytes to the laboratory, current equine in vitro maturation (IVM) systems achieve nuclear maturation rates of around 60% for both abattoir- and OPU-derived oocytes[21,24]. Whilst there are few comparative studies, and the sample sizes are often small, the accumulating evidence suggests that OPU-derived embryos have superior in vitro and in vivo developmental potential compared with abattoir-derived embryos[8,17,25]. To the best of our knowledge, only one equine SCNT study has directly compared the developmental competence of both types of oocytes, and in that study, there was no assessment of in vitro development because the embryos were transferred to recipient mares immediately after couplet activation[17].
Blastocysts are commonly cryopreserved in equine embryo transfer programs when recipient mares are unavailable[26], and commercially acceptable pregnancy and foaling rates have been achieved following the transfer of cryopreserved equine embryos produced by ICSI[27,28]. In relation to the transfer of cryopreserved equine blastocysts produced by SCNT, Galli et al.[8] observed no difference in pregnancy rates between fresh and frozen embryos, but there is a paucity of literature on the effects of vitrification on the full-term viability of SCNT equine embryos... To read the complete article you need to be a subscriber
CLICK HERE TO SUBSCRIBE TO BREEDING NEWS
SUBSCRIBERS CAN READ THE COMPLETE ARTICLE BY LOGGING IN AND RETURNING TO THIS PAGE